Mycoplasma Contamination in Cell Culture:
Causes, Detection & Removal
Mycoplasma are small, prokaryotic organisms, and are the most common contaminants found in cell cultures. Contamination by Mycoplasma continues to be a major issue in cell culture, as it can cause drastic changes in cell metabolism.
The presence of Mycoplasma influences the growth and proliferation of cells in culture negatively. They rob essential nutrients and cause diverse forms of cellular damage, which in turn may lead to significant errors in laboratory research results. It is believed that a large amount of cell lines nowadays are contaminated by Mycoplasma – often unknowingly.
Mycoplasma are the smallest, self-replicating organism discovered to date and easily pass through the typical sterile filtration processes, which are currently used to remove bacterial or fungal contaminants. As they have no cell walls, Mycoplasma are resistant to most common cell culture antibiotics that target cell wall synthesis, such as penicillin.
NEGATIVE IMPACT ON CELL CULTURES
Mycoplasma contamination can drastically change cell metabolism and therefore negatively impact research data.
NOT VISIBLE
A contamination by Mycoplasma is not directly visible under the microscope or by turbidity of growth media, and often remains undetected.
RESISTANT TO COMMON ANTIBIOTICS
Mycoplasma lack a cell wall around their membrane and can therefore not be eliminated with common cell culture antibiotics.
Quick Links
What is Mycoplasma Contamination?
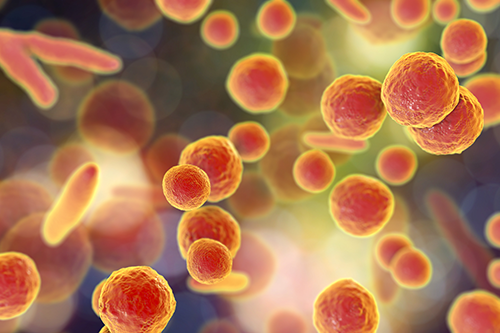
The Biology of Mycoplasma
Mycoplasma is a genus of bacteria that are characterized by the lack of a rigid cell wall, just like the other members of the class Mollicutes. In fact, Mycoplasma and mollicutes are commonly used as synonyms.
Mycoplasma are known as the smallest (0.3–0.8 µm), self-replicating organism on this planet. Further, they can shrink to diameters below 0.45 µm due to their flexible cell membrane, allowing them to pass through the typical antibacterial filters.
Their small size and the lack of a cell wall, which makes them resistant to common antibiotics (e.g., Penicillin/Streptomycin), are the reason why almost every cell culture scientist has to deal with them. It is believed that Mycoplasma are present in nearly every lab – and often remain undetected, unknowingly tampering with the scientific research data. Several large studies suggest that anywhere between 15–35% of continuous cell lines are affected by Mycoplasma contamination.
Effects of a Mycoplasma contamination
What happens when a cell culture is contaminated by Mycoplasma?
Mycoplasma are extremely simple organisms and, due to their parasitic nature, rely on eukaryotic host cells for metabolism needs. In the early stages, Mycoplasma will attach to the host cell and eventually fuse with its membrane. After this point, the Mycoplasma will replicate until they outnumber the host cell by a 1000-fold.
The effects of Mycoplasma contamination may be very subtle and cannot be visualized like common bacterial contamination, as Mycoplasma do not cause turbidity in the growth medium.
Regardless, a contamination of cell cultures by Mycoplasma can result in:
- drastic changes in cell metabolism
- negative impact on cell growth & proliferation
- decrease in transfection rate
- altered gene expression & virus production
- chromosomal aberrations
- faulty synthesis of nucleic acids
- cell death
These effects, being different – depending on your cell line and the Mycoplasma species – pose a serious issue for cell culture scientists, as valuable scientific data may be tampered with.
What causes a Mycoplasma contamination?
Mycoplasma contamination is usually spread by cross-contamination in between cell lines. For instance, when you receive a cell line from another lab, they may have ongoing contamination without knowing it. Unfortunately, most labs do not test incoming cell lines – or their culture facility in general – for Mycoplasma.
The genus Mycoplasma has various species, yet the largest percentage of Mycoplasma found in contaminated cell cultures is of human origin. Other found species are of bovine (Fetal Bovine Serum and Newborn Calf Serum) and porcine origin (trypsin sourced from swine), yet the impact of these sources is decreasing due to advancements in quality of product and manufacture.
So, if humans are the biggest source of Mycoplasma contamination, how do they cause it?

Mycoplasma contamination caused by lab personnel
The major source of Mycoplasma contamination is assumed to be the laboratory personnel. By coughing, sneezing – or simply talking – humans generate aerosols that carry various Mycoplasma. The contamination can then occur directly in the cells, or within any lab reagent or equipment, e.g., culture media, serum, incubators, water baths. The possibilities are endless.
As mentioned, Mycoplasma contamination can spread via cross-contamination in between cell cultures. Once you have a contaminated culture in an incubator, it will spread among all other cultures present – possibly extending to the whole cell culture lab. This is often facilitated by poor lab practices, such as an improper aseptic technique when handling serum, media, and other reagents. Unfortunately, the cause can also be as menial as aerosols that are created by pipetting.
As persistent and sturdy organisms, they can even survive on surface areas anywhere in the bench for numerous days! Since Mycoplasma are very small, they often remain undetected for a long time and could eventually become a cell culturist's worst nightmare.
How to detect Mycoplasma contamination?

The lack of a cell wall and how Mycoplasma attach themselves to their host cells makes them invisible to the naked eye, even with microscopy. Also, Mycoplasma do not cause turbidity in the growth medium. Only by observing your cells very carefully, you may notice changes in proliferation, morphology, or transfection efficiency – yet at this point, you would most likely discard your culture.
As of today, there are many ways to detect Mycoplasma contamination, and each of them may have their particular advantages and disadvantages.
European Medicines Agency (EMA) methods
The European Medicines Agency (EMA) offers guidelines for the detection of Mycoplasma contamination, which you can use as a reference. The microbiological culture method is their gold standard, where a liquid medium is inoculated with your sample, and later grown on Mycoplasma agar plates.
Another EMA-recommended method is DNA staining by fluorochromes (e.g., DAPI), yet interpreting the results can be very tricky and easily lead to misinterpretation, especially if your culture is in a poor condition.
Detection by PCR
If you are looking for a simple, low-budget solution for Mycoplasma detection, PCR may be your go-to method. There are plenty of commercial PCR kits available on the market, offering a sensitive and rapid method for Mycoplasma detection.
Preventing Mycoplasma contamination

Although it can be tricky to identify and eliminate a Mycoplasma contamination, there are some simple practices you can follow, in order to prevent it – most of which are good lab practices anyway!
Incoming cell lines
When you receive a new cell line from an external source, it is important that you quarantine it for a few weeks and test for Mycoplasma. This will prevent introducing a possibly contaminated cell culture into your lab. Once deemed safe, you can also keep a seed stock to fall back on, should you face contamination.
Aseptic technique
Next, it is crucial that you make use of proper aseptic techniques and wear PPE (gloves, clean lab coat, mask) whenever working in your cell culture. Even a dirty lab coat or streetwear can be the origin of your Mycoplasma contamination!
Use of antibiotics
Do not indiscriminately use antibiotics to prevent contamination, as Mycoplasma are resistant to the standard antibiotics (e.g., penicillin, streptomycin). This will merely act like a mask: bacteria that cause turbidity are eliminated, while Mycoplasma survive without detection.
Reagents & equipment
Ensure all of your cell culture reagents (sera, media, supplements, etc.) are free of Mycoplasma by choosing a trustworthy supplier. Also make sure your laminar-flow hood, incubator, and other equipment undergo regular inspections.
Conclusion
In conclusion, it will largely depend on your lab's cell culture routine, whether you can prevent Mycoplasma contamination. Yet, there will never be a 100% guarantee. Ensuring proper personnel training and continuously optimizing the process (even for the worst-case scenario) will go a long way. Work together to prevent contamination by testing regularly – an early detection can be a game-changer!
Mycoplasma Removal
Once contaminated with Mycoplasma, the best practice would be to discard your cell culture and start from scratch with fresh stock – after detecting and eliminating all other sources of contamination!
Unfortunately, this is not always possible: your cell culture may be irreplaceable, or you simply do not have the time. In these cases, Mycoplasma can be eliminated by following methods:
- physical (e.g., autoclave)
- immunological & chemical
- chemotherapeutic methods (e.g., antibiotics)
To this day, there is no one-size-fits-all approach to Mycoplasma removal, as the choice of method will largely depend on your cell line and Mycoplasma species. Also, Mycoplasma may develop antibiotic resistance due to improper use of cell culture antibiotics. Nevertheless, antibiotic treatment has been proven to be the suitable approach in most cases, the three groups of antibiotics being macrolides, tetracyclines, and quinolones.
When administering antibiotics for Mycoplasma removal, it is important that the treatment is done with sufficient duration and reagent concentration. Keep your cells quarantined to avoid spreading, and closely observe eradication by testing. Finally, it is important to complete the process with additional test methods, as the contamination may simply have fallen below detection levels of your initial assay.
Frequently Asked Questions (FAQ)
What are Mycoplasma?


Mycoplasma are small, microorganisms without a rigid cell wall that proliferate in vertebrates, in both parasitic and largely commensal manners. As facultative anaerobes, their temperature optimum is 37 °C. The lack of cell wall is responsible for the ineffectiveness of standard antibiotics (e.g. penicillin, streptomycin, gentamicin) and thus represents one of the main sources of contamination in cell culture.
See also "The biology of Mycoplasma".
Can I detect Mycoplasma contamination with the naked eye?


Mycoplasma contamination is difficult to detect by eye. Moreover, they can easily pass through standard filters. Visible indicators such as turbidity or pH changes are rare. Detection of Mycoplasma by analytical methods is therefore essential. The most sensitive method is therefore the direct culture method.
What is the effect of Mycoplasma contamination on my cell culture?


Mycoplasma are parasites that obtain important nutrients not only from the cell culture medium, but also from cells. The release of toxins damages the cultured cells.
Detectable effects are:
- Reduction of host cell metabolic activity.
- Inhibition of cell proliferation due to nutrient deficiency
- pH changes due to glucose degradation
- Inhibition of differentiation
- Alteration of membrane properties and protein expression profile
- Chromosome aberrations
- Arginine depletion
- Insensitivity to antibiotics, e.g., penicillin, streptomycin
- Impairment of the transfectability of cells
- Modulation of cellular cytokine production
For more information, see also "Effects of a Mycoplasma contamination".
Why is Mycoplasma contamination a problem?


In addition to the problems already listed above regarding the metabolic activities of the cell, identification of Mycoplasma contamination is extremely difficult. Macroscopic detection using a microscope is not possible. Also, Mycoplasma kill their host cells very slowly, which affects the live cell count only at a later stage. Another problem is the decade-long presence of Mycoplasma in cell lines, which could falsify important research results.
Why should I test my cell cultures for Mycoplasma?


An increasing number of established cell lines are showing Mycoplasma contamination. Currently, 15-35% of cultures in cell culture banks are found to be contaminated. An even higher number of unreported cases in selected populations is assumed. Less than 20% of laboratories regularly test for Mycoplasma.
So before using a cell culture from another lab, test it for purity. This provides assurance that the cell culture is sterile and free of contamination.
See also "Effects of a Mycoplasma contamination".
What are possible causes of contamination?


In general, a distinction is made between direct and indirect contamination.
Direct contamination usually occurs through the addition of contaminated media or animal products (e.g. FBS, growth factors, coating proteins). Indirect contamination is mostly caused by non-sterile working techniques or lack of quality control.
The use of antibiotics makes it difficult to detect bacterial contamination and Mycoplasma. Due to this, transfer/cross-contamination between cell lines is frequently encountered.
For further information, see also "What causes a Mycoplasma contamination".
How long do Mycoplasma survive?


As persistent and robust organisms, they can survive for several days on surfaces and sterile workbenches. Within a media droplet, their lifetime can even be up to 6 weeks at RT.
How often should I test my cell culture for Mycoplasma?


We recommend testing every 1-2 months.
Regular testing is essential, especially during the use of antibiotics. Standard antibiotics (e.g. penicillin) lead to the elimination of bacteria with cell walls, but are not effective against Mycoplasma. Thus, contamination resulting from non-sterile working techniques does not cause turbidity. It appears that the cell culture is sterile, but still has Mycoplasma.
My cell culture is contaminated with Mycoplasma, what can I do?


In case of contamination, the infected cell culture should be discarded and a new stock thawed.
To date, there is no patent remedy for the removal of Mycoplasma; the choice of method depends on the particular cell line and, if present, antibiotic resistance.
Nevertheless, antibiotic treatment with macrolides, tetracyclines and quinolones has been proven to be suitable.
You can find everything you need to know here: "Mycoplasma Removal".
Are Mycoplasma from cell culture transmissible to humans?


Yes, transmission to humans is possible.
Mycoplasma can survive both commensally and parasitically in humans. In the human body, they live on the surface of epithelial cells or in the gastrointestinal tract. Pathogenic species are mainly transmitted via droplets. By changing their surface proteins with high frequency, they are difficult for the immune system to detect. Among other things, mycoplasmas are associated with atypical pneumonia.
How should samples be stored, and can I directly test thawed cells for Mycoplasma?


Optimally, the supernatant should be processed directly. If the samples are processed within one day, storage at 4 °C is required.
Alternatively, the samples can be stored at -80 °C for up to 6 months. It is important that the supernatant is frozen immediately after centrifugation. Thawing of the supernatant (without exposure to heat) for at least 15 minutes at room temperature is recommended prior to testing.
How can I detect Mycoplasma analytically?


Due to the lack of a cell wall, macroscopic detection is very difficult. Commercial kit systems or published standard methods are suitable for checking cell cultures.
The main methods used for the detection of Mycoplasma contamination are:
- Mycoplasma cultures:
A special liquid medium is inoculated with your sample and later grown on Mycoplasma agar plates.
- DAPI or Hoechst staining:
DAPI (4',6-diamidino-2-phenylindole) intercalated with the DNA of the Mycoplasma leads to a blue staining which can be visualized by UV excitation (460 nm) using a fluorescence microscope. Negative cell cultures show only a blue staining of the cell nuclei.
- PCR:
The polymerase chain reaction (PCR) leads to amplification of the bacterial DNA, i.e. an amplification of the Mycoplasma DNA. PCR allows routine detection at early stages and at low concentrations.
The European Medicines Agency (EMA) offers guidelines for the detection of Mycoplasma contamination.
For more information, see also "How to detect Mycoplasma contamination?".
What starting material do I need for testing?


In most cases, cell-free culture supernatant from cultures incubated for 48-72 h is used for testing. It is therefore important that the medium is not changed or discarded during cultivation. The culture supernatant is then centrifuged so that no cell residues remain. Cell residues would lead to a falsification of the results.
Instructions for Use
How to detect Mycoplasma contamination: Direct culture method


Direct culture method
If detection of live Mycoplasma in the medium to be examined is required, use detection by direct culture method. A distinction is made between liquid cultures and nutrient agar plates. Incubate the inoculated culture media under microaerophilic conditions at 35-38 °C with 5-10 % CO2.
Reference controls:
- Positive control: uninoculated liquid culture.
- Negative control: uninoculated agar plate
Sample preparation:
Nutrient agar plate (e.g. MU agar):
Selective nutrient media specific for mycoplasma growth are inoculated with 0.2 ml of the sample to be tested (< 100 CFU). Subsequent cultivation is performed at 35-38 °C under microaerophilic conditions. Note that sufficient humidity is ensured. If your medium shows bacterial or fungicidal contamination - repeat your assay.
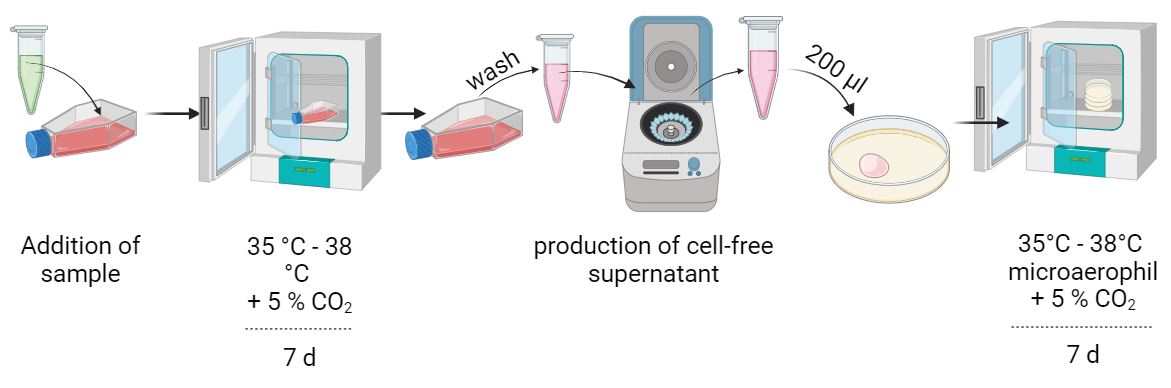
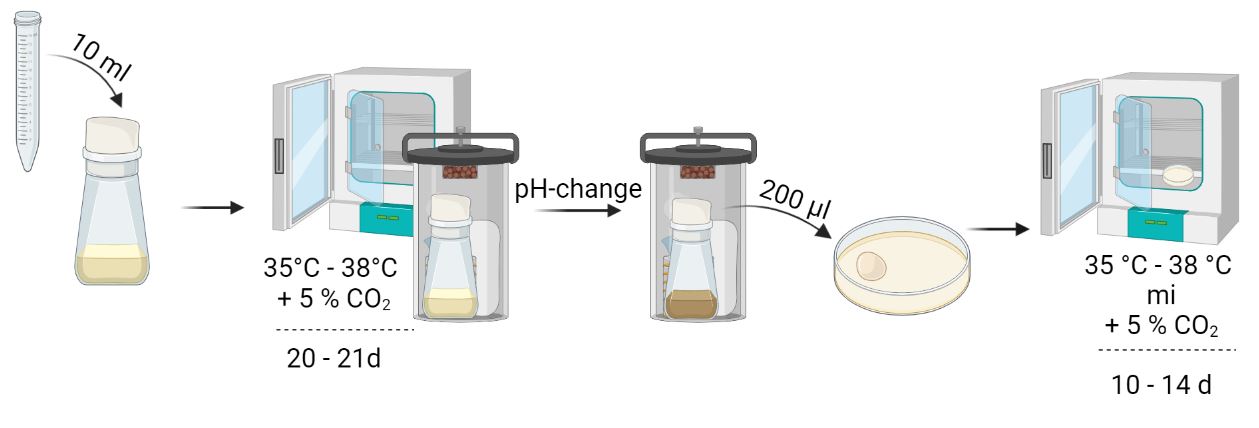
Liquid culture (e.g. Hayflick, PPLO):
Liquid cultures are particularly well suited for master or working batches, as well as for testing components of animal origin. For this cultivation approach, use a minimum volume of 10 ml of the undiluted sample and add this to your liquid culture. Subsequent cultivation is performed at 35-38 °C in tightly sealed culture vessels for 20-21 days. On day 2 and 4, inoculate a subculture with 0.2 ml of the suspension and culture at 35-38 °C under microaerophilic conditions for 10-14 days. Repeat this procedure on day 6, 8, 13, 15, 19, and 21. Passages cultured on day 19 and 21 are incubated for 7 days. A pH change of the medium is causative for a color change of the medium. As soon as this occurs, a subculture should be started.
Evaluation
The solid media are evaluated microscopically for the presence of Mycoplasma colonies. The sample is considered negative if the growth of typical Mycoplasma colonies cannot be detected on any of the inoculated solid media.
Suspect colonies may be confirmed by appropriate and validated methods. The test is invalid if:
- No growth is seen on the positive control or
- Growth is seen on the negative control
How to detect Mycoplasma contamination: DAPI staining


DAPI staining
A simple way to test for the presence of Mycoplasma in your cell culture is to use DAPI staining. DAPI (4',6-diamidino-2-phenylindole), is a fluorescent dye that intercalates with AT-rich region of mycoplasma DNA. This results in a blue staining that can be visualized by UV excitation (460 nm) using a fluorescence microscope.
Sample preparation
Culture the indicator cell line in a 25 cm2 cell culture flask (glass bottom dishes, confluency 60-80 %). Passage the cell line as usual, without using an antibiotic. Then add 1 ml of your sample to the cell culture and culture the cells for three days at 35-38 °C.
Protocol
Fix and permeabilize the cultured cells using a protocol suitable for your samples. The performance of DAPI staining differs depending on the protocol and experimental setup. A detailed execution from the EMA can be found here.
Example of execution
- Remove the cell culture medium
- Incubate the cells with 4 % PFA for 15 min at RT
- Wash the cells 2 times with 1x PBS
- Add the DAPI solution (1:5000 dilution)
- Incubate for 1 min in the absence of light
- Wash the cells 2-3 times with 1x PBS
- Resuspend the cells in 1x PBS
- Fluorescence microscopic evaluation at 400x magnification
Evaluation
Compare your microscopic image with your reference controls and check for extranuclear florescence. Mycoplasma form characteristic filamentous structures over the cytoplasm of the indicator cell. In addition, they can produce filaments in intracellular spaces. A sample is considered negative if no expressions characteristic of Mycoplasma are detected.
The test is invalid if:
- No growth is seen on the positive control or
- Growth is seen on the negative control
How to detect Mycoplasma contamination: PCR detection


PCR detection
This method is based on the specific amplification of a DNA segment of the Mycoplasma 16S rRNA gene. Total DNA is extracted from the obtained cell culture supernatant using designated DNA extraction and purification test kits or other methods. PCR allows routine detection at early stages and use of low concentrations. In addition, detection of live and dead mycoplasmas is performed. For further specification of the detected DNA, further restriction analysis or sequencing of the PCR product is required. All work is to be performed in an S2 laboratory and under safety cabinets.
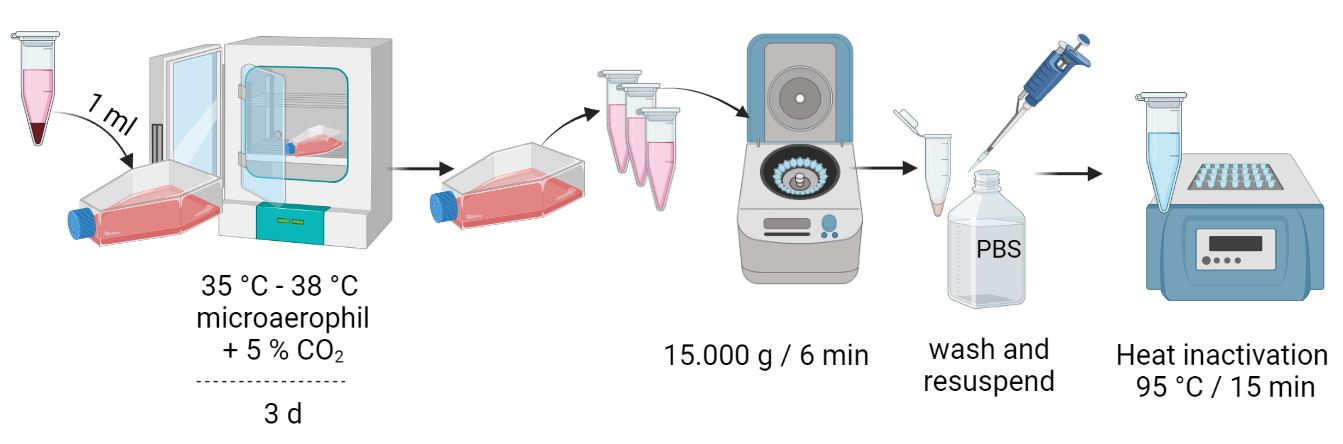
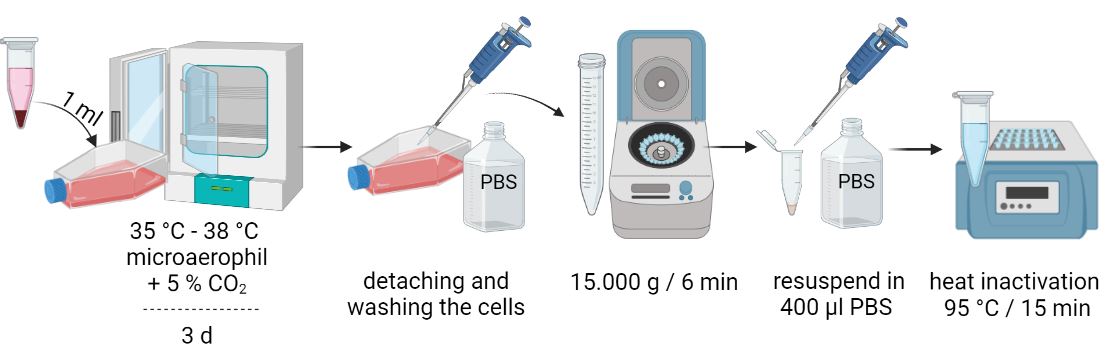
Sample preparation
Culture the cell line to be analyzed in a 25 cm2 cell culture flask until confluent growth (60-80 %) has been achieved. Passage the cell line for further analysis (1/5 at 90 % confluence) and take at least 3 ml supernatant per cell culture in the meantime. If the analysis is not performed on the same day, it is best to store your samples at a minimum of 4 °C.
Protocol
From cell culture supernatants
- Centrifuge 1 ml cell culture supernatant at 15,000 x g for 6 min.
- Discard supernatant
- Wash pellet 2x with 1 ml each of 1x PBS buffer
- Centrifuge at 15,000 x g for 6 minutes
- Resuspend cell pellet in 200 µl PBS
- Inactivate the suspension for 15 minutes at 95 °C
- Sample input for DNA extraction: total sample volume
Cell pellets from cell cultures
- Discard supernatant
- Wash 80 % confluent cells 2x with PBS
- Dissolve adherent cells with trypsin for 5 minutes beforehand
- Inactivate trypsin by adding 5 ml culture medium
- Centrifuge suspension mixture for 5 minutes at 200 x g
- Resuspend cell pellet in 1.8 ml PBS
- Centrifuge at 15,000 x g for 3 minutes
- Discard supernatant and resuspend cell pellet in 400 µl PBS
- Inactivate suspension for 15 minutes at 95 °C
- Sample input for DNA extraction: 200 µl
DNA extraction and PCR
Commercially available extraction test kits and analyzers are suitable for DNA extraction from cell cultures and for PCR analysis. For further procedure, please contact your respective manufacturer or send your samples to an analytical laboratory of your confidence.
Preparing different dilutions of MycoXpert


MycoXpert is provided as a 50x concentrate.
Recommended working concentration: 1x.
Therefore, dilute the stock solution 1 : 50 (Dilution 1)
Example: Add 10 ml MycoXpert to 500 ml base medium.
If required, increase MycoXpert concentrations as follows in different subsequent steps:
Dilution 1 → 1 : 50
Dilution 2 → 1 : 40
Dilution 3 → 1 : 30
Dilution 4 → 1 : 25
Eliminating Mycoplasma contamination using MycoXpert


For the different concentrations, please see "Preparing different dilutions of MycoXpert".
Use the following protocol, starting with Dilution 1 (1:50):
- Remove culture medium from culture vessels, wash cells with PBS and detach cells using Trypsin or Accutase.
- Count cells and plate in fresh medium (with MycoXpert).
- Cultivate cells for 2 to 3 days in the usual way.
- Repeat the passaging cycle at least twice.
- Check for Mycoplasma contamination (Staining method, cultivation or ELISA).
- If mycoplasma positive, check methods (positive and negative controls and evaluate).
- If required, repeat treatment with increased MycoXpert concentrations (Dilution 2, Dilution 3 or Dilution 4).
MYCOXPERT: BENEFITS AT A GLANCE
- highly effective antibiotic
- works even in very low concentrations
- suitable for a wide spectrum of Mycoplasma species
- interferes with the DNA machinery of Mycoplasma
- no negative effects on proliferation of your cells
- very simple to use
- cost-effective solution for Mycoplasma removal
MycoXpert downloads
PRODUCT SPECIFICATIONS
| Appearance | Clear frozen liquid |
|---|---|
| Storage and shelf life | (long term, ≤ -15 °C) (Once opened, store at +2 to +8 °C. Use within 4–6 weeks.) Avoid repeated freeze-thaw cycles. Preparation of aliquots recommended. |
| Shipping conditions | (dry ice) |
| Thawing | Overnight at +2°C to +8 °C. Swirl gently to homogenize. |
| Working concentration | 1x (recommended) |