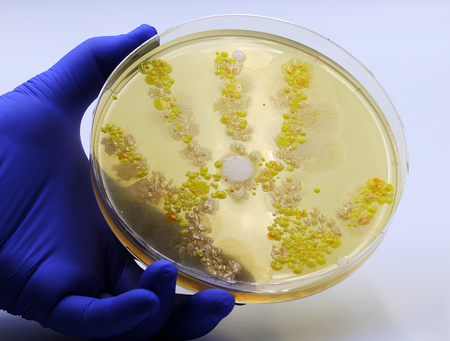
Cell Culture Contamination: 5 Common Sources and How to Prevent Them
Contamination is one of the most common and frustrating challenges in cell culture. Whether you're working in academic research or biotech production, a single contamination event can compromise months of work.
In this article, we’ll explore the top 5 sources of contamination in cell culture and offer practical, actionable steps to prevent them — helping you maintain the integrity and reproducibility of your results.
Reviewed by Dr. Sabrina Friederichs · Last updated June 18, 2025
TL;DR: Summary
Preventing Contamination in Cell Culture
If you only have 30 seconds, here’s what matters most:
- Use strict aseptic techniques — every single time
- Avoid relying on antibiotics — they hide more than they help
- Test for contamination regularly, especially mycoplasma
- Handle only one cell line at a time and label everything clearly
- Keep your incubators and workspaces clean and well-maintained
- Use certified, contamination-free reagents
- Quarantine and verify all new cell lines before use
Free Printable Checklist: Keep Your Cultures Clean
Download our contamination prevention checklist and post it right at your workbench!
- ✅ Daily handling reminders
- 🧪 Weekly and monthly maintenance logs
- 🔬 Key checkpoints for mycoplasma, cross-contamination & more
📋 Overview of Contamination Risks in Cell Culture
Jump to a specific section:
1. Bacterial Contamination
An Invisible Threat with Visible Consequences
Bacteria are one of the most common and rapidly destructive sources of contamination in cell culture.
They can enter cultures through unclean surfaces, contaminated reagents, or poor aseptic technique — and their effects are often fast and noticeable.
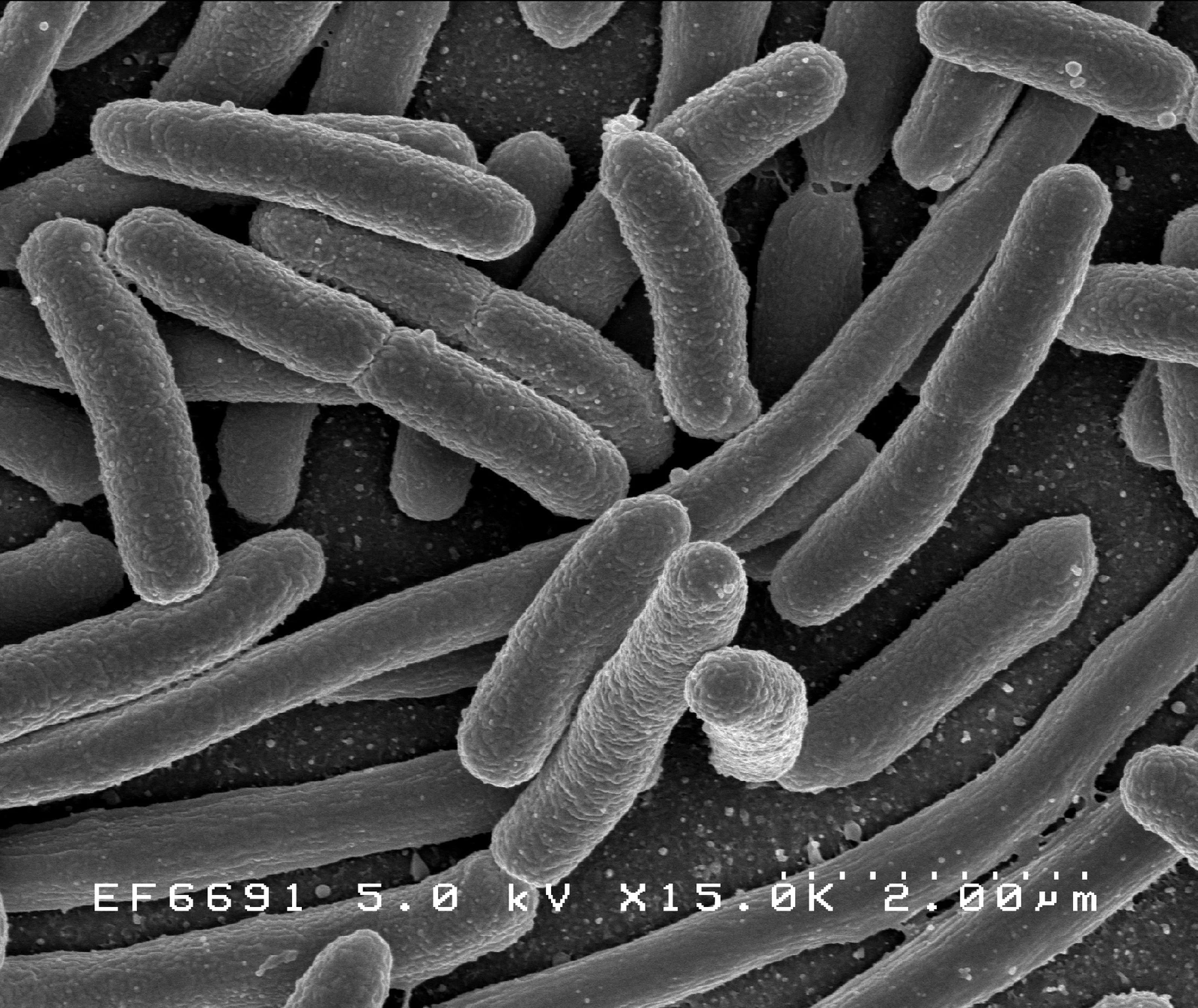
🔍 How to Recognize Bacterial Contamination
- Cloudy or turbid culture medium
- Sudden drop or fluctuation in pH (e.g., medium turns yellow)
- Unpleasant or sour odor
- Unusual cell morphology or slowed growth
- Small (∼1–5 µm), motile particles visible under the microscope
✅ How to Prevent Bacterial Contamination
- Always work under a properly maintained laminar flow hood
- Use sterile pipette tips, flasks, and reagents
- Disinfect work surfaces before and after each session (70% ethanol or similar)
- Limit antibiotic use to avoid masking low-level contamination or promoting resistance
- Store reagents properly and check expiration dates regularly
💡 Understanding the Risk
How It Spreads
Bacterial contamination can quickly compromise an entire batch of cell cultures. Itʼs usually introduced through improper handling, poor cleaning routines, or overlooked contamination in reagents. Once inside, bacteria multiply fast, outcompeting the cultured cells and altering their environment — often without a chance for recovery.
Why Prevention Matters
While visual signs like turbidity make detection relatively easy, prevention is more effective than damage control. Many labs rely on antibiotics as a safeguard, but this can create a false sense of security. Instead, consistency in aseptic technique, attention to environmental cleanliness, and proper reagent handling form the backbone of bacterial contamination control.
2. Mycoplasma Contamination in Cell Culture
The Invisible Danger
Due to their small size (~0.3 µm) and lack of a cell wall, species of Mycoplasma are a major threat in cell cultivation and often remain undetected.
🔍 How to Detect Mycoplasma Contamination
- No visible signs in the medium (no cloudiness or odor)
- Unexplained changes in cell growth rate or morphology
- Reduced transfection efficiency
- Confirmed through specific tests:
- PCR (high sensitivity, Protocol on PubMed)
- Fluorescence staining
- ELISA or DNA-binding dyes
✅ How to Prevent Mycoplasma Contamination
- Use only certified Mycoplasma-free cell lines and reagents
- Quarantine and test all new cell lines before integrating them
- Perform routine Mycoplasma screening (e.g. every 1–2 months)
- Purchase FBS and supplements from reliable, tested suppliers
- Avoid sharing media or equipment between different cell lines
- Maintain rigorous aseptic technique and biosafety training
💡 Why Mycoplasma Is So Dangerous
Why It’s Hard to Detect
Unlike other bacteria or fungi, Mycoplasma doesn't cause visible turbidity or produce odor. It slips past routine visual checks, making it one of the most deceptive contaminants in cell culture. Because it lacks a cell wall, it's resistant to many standard antibiotics and can pass through typical filters used for sterilization.
Why It’s So Harmful
The real damage lies in its subtle interference: altering DNA, inhibiting cell division, or modifying cytokine production — often without you realizing it until your experimental data is inconsistent or irreproducible. Once contamination is confirmed, eradicating it without sacrificing the entire cell line is extremely difficult.
That’s why prevention and early detection are non-negotiable. Regular screening, strict sourcing policies, and meticulous technique are your best tools against this invisible threat.
👉 Want to Learn More About Mycoplasma Contamination?
Check out our in-depth article:
Mycoplasma Contamination – Everything You Should Know
It covers detection methods, sources, and eradication strategies in full detail — ideal if you're dealing with a suspected contamination or want to build a long-term prevention strategy.
3. Filamentous Fungi and Yeasts
Airborne Threats to Cell Cultures
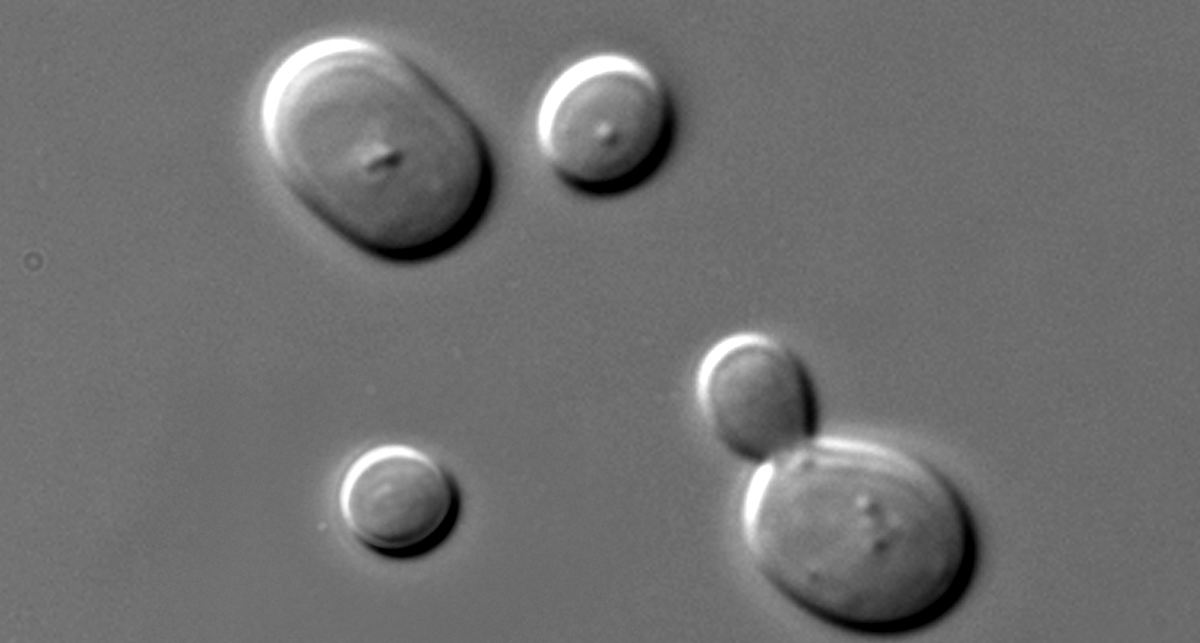
Filamentous fungi and yeasts grow more slowly than bacteria but still much faster than cell cultures, making them persistent and aggressive contaminants. They often sneak into cultures via airborne spores, poorly maintained equipment, or contaminated reagents. Unlike bacterial contamination, fungal outbreaks may take longer to notice — but once established, they can be much harder to eliminate.
🔍 How to Recognize Fungal/Yeast Contamination
- Filamentous threads or "fuzzy" structures floating in the medium
- Visible colonies (white, green, or dark patches) on flasks or dish surfaces
- Changes in medium clarity or surface tension
- Fermented odor of the cell cultures
- Microscopic hyphae or budding cells (~10 µm) visible during routine inspection
✅ How to Prevent Fungal/Yeast Contamination
- Ensure proper air filtration (HEPA filters) in culture rooms and hoods
- Decontaminate CO₂ incubators weekly — including shelves, door gaskets, and water trays
- Monitor and regulate humidity, especially in warm environments
- Inspect cultures microscopically on a regular schedule
- Avoid opening bottles and flasks outside of laminar flow conditions
- Replace contaminated water pans immediately — a frequent but overlooked source
💡 Why Prevention Matters
Fungal Spores Linger Everywhere
Filamentous fungi and yeasts often fly under the radar until they visibly colonize surfaces or interfere with cell viability. Unlike bacteria, they can form spores that survive on surfaces or in the air for long periods, making them particularly resilient. If your incubator water tray, humidifier, or even lab coat is a little too friendly to spores, the entire culture environment becomes vulnerable.
Vigilance Beats Cleanup
Controlling humidity and maintaining strict air quality are key lines of defense. But vigilance during routine procedures — like inspecting cultures and cleaning surfaces — is what truly keeps fungal threats at bay. Once a fungal colony appears, full sterilization and discard of affected cultures are usually the only safe options.
4. Cross-Contamination
An Underestimated Risk
Cross-contamination happens silently but can permanently compromise your cell culture work.
It occurs when cells from one line infiltrate another — usually due to simple handling mistakes or poor lab routines. Since most cross-contamination isn't immediately visible, it often goes undetected until inconsistencies arise in your data.
🔍 How to Detect Cross-Contamination
- Unexpected changes in cell behavior or morphology
- Inconsistent experimental results across passages
- Authentication mismatch through:
- STR profiling (Short Tandem Repeat analysis, Article in Nature)
- DNA barcoding or isoenzyme analysis
✅ How to Prevent Cross-Contamination
- Handle only one cell line at a time
- Use dedicated pipettes, media, and reagents for each line
- Clearly and consistently label all vessels (cell line name, date, passage number)
- Implement routine cell line authentication (e.g. every 6–12 months)
- Establish and follow SOPs for culture handling and labeling
- Avoid splitting attention between tasks — stay focused on your workflow
💡 Why This Risk Is Often Overlooked
It Doesn’t Look Like Contamination
Unlike microbial contamination, cross-contamination doesn’t cloud your medium or emit strange smells — instead, it quietly invalidates your research. A more aggressive cell line can outcompete a slower one, leading to overgrowth, altered phenotype, and false data.
It’s Often Caused by Workflow Pressure
In academic and industrial labs alike, cross-contamination often stems from time pressure or multitasking. Switching between cultures, using the same reagents or pipettes across lines, or simply forgetting to relabel a flask can introduce foreign cells into a supposedly pure culture.
Routine authentication isn’t just for publication requirements — it’s a frontline defense. Combined with strict procedural discipline, it helps ensure that your “HeLa” line is still HeLa... and not something else entirely.
Get expert tips, new protocols & prevention tools — straight to your inbox.
Join Our Newsletter
5. Viral Contamination
A Risk to Research and Production
Viruses are among the most serious — and most difficult — cell culture contaminants to manage.
They can silently infect cultures without obvious signs, remain latent for extended periods, and pose significant safety concerns in both academic and industrial settings. Especially in biopharmaceutical production, viral contamination can lead to halted projects, product recalls, and regulatory consequences (Learn More: Article in Nature).
🔍 How to Detect Viral Contamination
- No visual indicators in many cases — latent viruses may not affect medium appearance
- Unexplained cytopathic effects (cell detachment, rounding, or syncytia)
- Reduced productivity in bioprocessing (e.g. lower protein yields)
- Confirmed via:
- qPCR or RT-PCR
- Immunofluorescence or ELISA
- Electron microscopy in advanced cases
✅ How to Prevent Viral Contamination
- Use virus-screened sera or switch to chemically defined, serum-free media
- Test and quarantine all new cell lines before use
- Maintain strict biosafety protocols for all lab personnel
- Avoid sourcing materials from unverified suppliers
- Implement batch tracking and clear documentation for all incoming reagents
- Educate staff on human-mediated risks (e.g. respiratory viruses)
💡 Understanding the Long-Term Risks
Viruses Don’t Always Show Themselves
Unlike other forms of contamination, viruses can stay hidden — silently altering your cells or integrating into their genomes. Some viral infections may only affect sensitive readouts, like transcriptomic or proteomic analyses, while others can completely derail an entire cell-based production system.
The Price of Overlooking Prevention
The stakes are even higher in GMP and production settings, where viral contamination can invalidate an entire run, cost hundreds of thousands of euros, and damage reputation. Prevention, therefore, must be built into every stage — from raw material sourcing to staff training and sample handling.
Regular testing is expensive, yes — but not testing can cost far more.
Antibiotics in Cell Culture: Helpful or Harmful?
While not a direct contamination source, antibiotics can interfere with your prevention strategy — sometimes doing more harm than good.
Antibiotics and antimycotics are often used to reduce the risk of microbial contamination — but in most routine cell cultures, they can introduce new problems that undermine experimental integrity. While they may appear to help in preventing cell culture contamination, they often create a false sense of security that allows hidden contaminants to persist and distort results.
❌ Why Antibiotics Can Be Problematic
- They mask contamination, especially slow-growing infections like mycoplasma. Cultures may appear fine while contamination quietly spreads — making detection difficult until experimental results are already compromised.
- They promote resistant microbial strains over time. Bacteria and fungi exposed to sub-lethal antibiotic levels may adapt, making future infections harder to treat and increasing the risk of persistent lab-wide contamination.
- They alter cell behavior, even at low concentrations. Common antibiotics like penicillin-streptomycin can affect gene expression, stress response, and cell proliferation — skewing data and limiting reproducibility.
(Source: Nature Publication)
✅ When to Use Them — If At All
Antibiotics can be helpful in short-term situations — such as when establishing primary cultures from tissue (which carry a higher contamination risk, such as urine) or attempting to rescue a rare or valuable cell line. In these cases, their use should be strictly limited in duration and paired with frequent contamination testing (e.g., PCR for mycoplasma) to monitor effectiveness.
For all standard workflows, the best approach to preventing cell culture contamination is to skip antibiotics entirely and rely on robust aseptic technique, clean working environments, and verified, contamination-free reagents.
Conclusion: It’s Not Magic – Just Good Habits
Preventing contamination in cell culture takes more than just being careful — it’s about building smart habits into your daily workflow. The good news? Once you’ve got the right tools and routines in place, keeping your cultures clean becomes second nature.
✅ Key Takeaways to Prevent Cell Culture Contamination
- Test regularly – especially for silent threats like mycoplasma
- Use sterile, certified reagents – don’t take shortcuts with media or sera
- Stick to aseptic technique – consistency beats improvisation
- Clean your incubators and hoods – it’s easy to skip, costly if you do
- Handle one cell line at a time – and label everything clearly
- Train everyone – most contamination comes from people, not products
Tired of Contamination Wrecking Your Experiments?
Whether it's unreliable results, wasted time, or mysterious culture crashes — contamination always hits when you least expect it.
That’s why we offer tested, contamination-free cell culture reagents and expert support to help you prevent problems before they start.
👉 Contact us to keep your cultures clean — and your data trustworthy.
Free Printable Checklist: Keep Your Cultures Clean
This printable cheat sheet summarizes key tips from the article — perfect to hang in your lab for daily reminders.
- ✅ One-page summary of daily & weekly routines
- 🔬 Checklist fields to log cleaning and tests
- 📌 No email required – just print and post
Frequently Asked Questions (FAQs)
What are the main sources of contamination in cell culture?


The most common sources include bacteria, fungi, mycoplasma, viruses, and cross-contamination from other cell lines. These often enter cultures through poor aseptic technique, unclean equipment, or non-sterile reagents.
How can I prevent mycoplasma contamination?


Always quarantine and test new cell lines before use. Avoid relying on antibiotics, and regularly screen your cultures using PCR or ELISA kits. Proper aseptic technique is your best defense.
Is it safe to use antibiotics in all cell cultures?


Routine use of antibiotics is discouraged. While they can suppress bacterial growth, they may mask low-level contamination and promote resistant strains. It’s better to eliminate the root cause through clean handling.
How often should I clean the incubator and hood?


Wipe down hoods and work surfaces at least once per week. Incubators should be cleaned thoroughly (shelves, gaskets, water trays) on a weekly basis. Always document maintenance activity in a visible log.
Why should I authenticate my cell lines?


Authentication ensures you're working with the correct cell type and avoids misidentification or cross-contamination. Short Tandem Repeat (STR) profiling is the most common method and should be performed regularly.