Should You Use Antibiotics in Cell Culture?
Balancing Contamination Risk and Cell Line Integrity
In many labs, antibiotics are routinely added to culture media as a safeguard against contamination. But is that precaution always justified? And what hidden costs might it carry for your cells?
A large-scale study examining over 2,700 cell lines found contamination in nearly 40% — including mycoplasma in 19% of cases (Uphoff & Drexler, 2005). Given that reality, it’s no wonder so many labs default to Pen-Strep, Gentamicin, or Amphotericin B — especially in shared incubators or when handling primary cells.
But these compounds aren’t neutral. From altered gene expression to masked infections, they can quietly distort your data — often without any visible warning.
This article clarifies when antibiotics are helpful, when they aren’t, and how to use them without compromising the integrity of your experiments.
Reviewed by Dr. Sabrina Friederichs · Last updated Aug 4, 2025
TL;DR: Summary
Should You Use Antibiotics in Cell Culture?
If you only have 30 seconds, here’s what matters most:
|
|
|
|
|
|
|
Best Practices Quick Guide (Free PDF)
Download our printable PDF with summary tables, usage guidelines, and best practice reminders — perfect for your next SOP update or lab training.
- ✅ Working concentrations for Pen-Strep, Gentamicin & Amphotericin B
- 📋 Quick-reference contamination guide
- 🧪 When to use antibiotics — and when to avoid them
📋 Overview: Antibiotic Use in Cell Culture
Jump to a specific section:
What Are the Common Antibiotics in Cell Culture?
In cell culture labs, a few antibiotics tend to show up again and again — often as default additives to minimize risk in routine work.
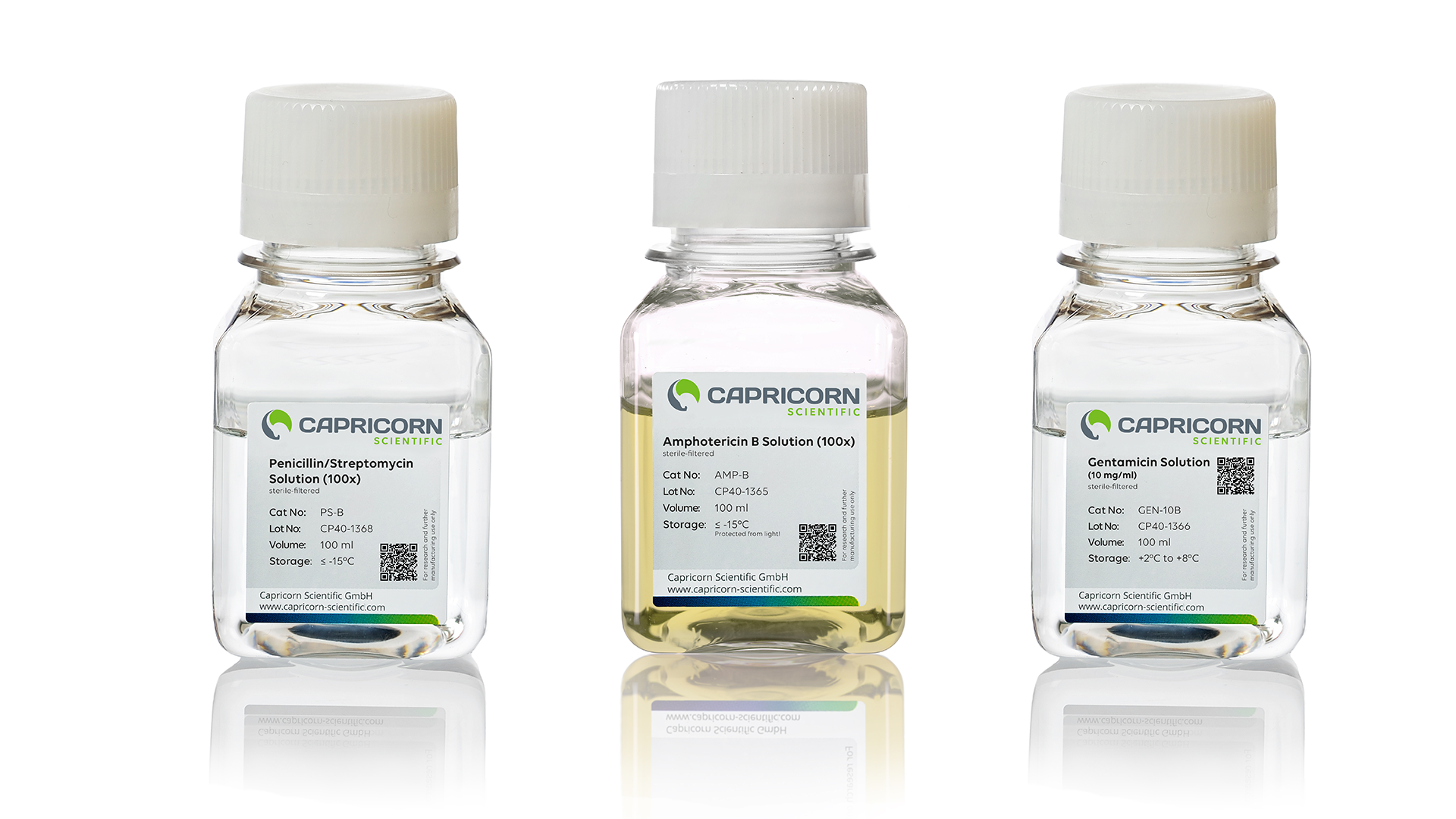
🦠 Penicillin-Streptomycin (Pen-Strep)
A long-trusted combination that targets a broad range of bacteria. Pen-Strep is especially common in busy labs where sterility is harder to control.
🧫 Gentamicin
Known for its broad-spectrum activity, Gentamicin is frequently included in pre-mixed antibiotic-antimycotic cocktails. It offers stronger coverage against Gram-negative strains — but may stress more delicate cell types.
🍄 Amphotericin B
This antifungal agent helps prevent contamination from fungi or yeast. It’s effective in small amounts, but higher concentrations can damage mammalian cells and require light protection during handling.
Note: This Article Covers Routine Antibiotics — Not Selection Agents
This article focuses on antibiotics used to manage contamination. It does not cover selection antibiotics like G418 or Hygromycin B, which are used to maintain stable transfectants.
That’s a topic for another day.
Pathogens and Recommended Antibiotics: What Works and What to Watch
Not all contaminants behave the same way — and no single antibiotic fits every threat. The table below outlines typical contaminants in cell culture and how they're commonly handled.
| Pathogen Type | Recommended Antibiotic(s) | Notes on Effectiveness / Cytotoxicity |
|---|---|---|
| Gram‑positive bacteria | Penicillin–Streptomycin (Pen‑Strep) | Synergistic combo; effective against most Gram-positives. Low cytotoxicity at standard 1× concentration. |
| Gram‑negative bacteria | Streptomycin, Gentamicin | Gentamicin offers broader Gram-negative coverage. Can stress sensitive cell lines. |
| Mixed bacterial flora | Pen‑Strep + Gentamicin | Covers a wider bacterial spectrum. Monitor for resistance with long-term use. |
| Fungal contamination | Amphotericin B | Effective antifungal. Higher doses can harm mammalian cells. Poorly water-soluble; typically formulated with deoxycholate to increase solubility. Light-sensitive — protect from light. Store at –20 °C. |
| Mixed fungus + bacteria | Antibiotic-Antimycotic solution (Pen‑Strep + Amphotericin B) | Convenient mix of Pen-Strep and Amphotericin B. For usage, cytotoxicity, and storage details, see individual components above. |
| Mycoplasma | — (Requires targeted treatment) Learn more here |
Lacks a cell wall — unaffected by typical antibiotics. Use PCR-based detection and Mycoplasma-specific elimination reagents. |
🧪 One of our customers once told us — during a late afternoon phone chat — about their early days in cell culture. Back then, they routinely used Pen-Strep “just to be safe.” When they finally tried removing it for a viability test, the entire culture collapsed within 48 hours. The silent contamination had gone completely unnoticed until the chemical crutch was gone.
Real-world stories like this show why antibiotics often hide problems instead of solving them.
Even when they reduce visible contamination, they can mask low-level issues. Combine antibiotics with proper technique, regular monitoring, and sterility checks.
Want more tips like this in your inbox?
Sign up for clear, practical updates on contamination control, cell culture strategy, and lab-tested workflows — made for real scientists, not marketers.
Subscribe to Our NewsletterAdvantages of Using Antibiotics
🧬 "We often treat antibiotics as a safety net — especially when thawing cells or working with primary cells that are more vulnerable to contamination."
— QC Manager, Capricorn Scientific
- Contamination Control:
Helps suppress bacterial and fungal overgrowth, especially in unpredictable or high-risk lab environments.
- Protection of Valuable Cell Lines:
Valuable or irreplaceable cultures benefit from added protection — particularly in shared incubators or busy workflows.
- Primary Cell Culture Support:
Primary cells are more susceptible during initial culture; antibiotics can provide temporary stability.
Risks and Drawbacks

Altered Gene Expression
Pen-Strep doesn’t just clear infections — it can subtly shift cellular behavior. In HepG2 cells, over 200 genes were differentially expressed when cultured with Pen-Strep, including those related to stress responses and metabolism.
(Source: Scientific Reports)
“Antibiotics don’t just protect your cultures — they quietly reprogram them.”
It’s a dramatic way to put it, but not far from the truth.
Cytotoxic Effects
High doses of Gentamicin or Amphotericin B can impair membrane function and slow proliferation — especially in fragile or stem-like cell types. Cell health should be monitored closely.
(Source: Gulhane Med J)
Masked Contamination
Antibiotics may hide problems rather than solve them. Instead of eliminating contamination, they often just suppress it — letting issues like mycoplasma persist unnoticed unless actively tested for. On top of that, prolonged use can affect cell behavior, as shown in keratinocyte studies.
(Source: Exp Dermatol. Nygaard et al., 2015)
Resistance Development
Overuse breeds resistance — even in the lab. One study found that more than 90% of bacterial isolates from contaminated cultures were resistant to Pen-Strep, echoing broader clinical trends.
(Source: Human Reproduction)
Antibiotic-Antimycotic Solutions & How to Use Them
Antibiotic-antimycotic mixes — typically sold as 100× liquid stocks — are a staple in many labs. Most combine:
- Penicillin (10,000 U/mL)
- Streptomycin (10 mg/mL)
- Amphotericin B (25 µg/mL)
These combinations offer a sense of security, especially in unpredictable lab environments — but they’re no substitute for solid aseptic technique.
As shown in the table above, this blend protects against both Gram-positive and Gram-negative bacteria, as well as fungal contaminants. Still, it's essential to recognize their limits.
Mycoplasma, for instance, has no cell wall — making it inherently resistant to most antibiotics, including those in mixed formulations.
Stock and Working Concentrations
| Antibiotic | Stock (Recommended) | Working Concentration | Notes | Solvent & Storage |
|---|---|---|---|---|
| Pen-Strep | 100× (10,000 U/mL) | 1× (100 U/mL / 100 µg/mL) | Synergistic; standard in most labs | Water-soluble; store at -20 °C; avoid repeated freeze–thaw cycles |
| Gentamicin Sulfate | 50 mg/mL | 10–50 µg/mL | Broad-spectrum; dose-dependent cytotoxicity | Water-soluble; store at –20 °C; stable in aqueous solution |
| Amphotericin B | 250 µg/mL | 0.25–2.5 µg/mL | Light-sensitive; higher doses may impact viability | Poorly water-soluble; typically formulated in colloidal suspensions with deoxycholate to increase its solubility. Light-sensitive — protect from light; store at –20 °C |
| Mycoplasma Removal Reagent | — | As directed by manufacturer | Targeted elimination; not a substitute for routine testing. Learn more about Mycoplasma contamination here. |
Typically stored frozen; may require aliquoting and light-sensitive handling. Always follow manufacturer instructions. |
Always check the product’s datasheet for solvent compatibility and shelf-life guidance.
Best Practices: When to Use & When to Avoid
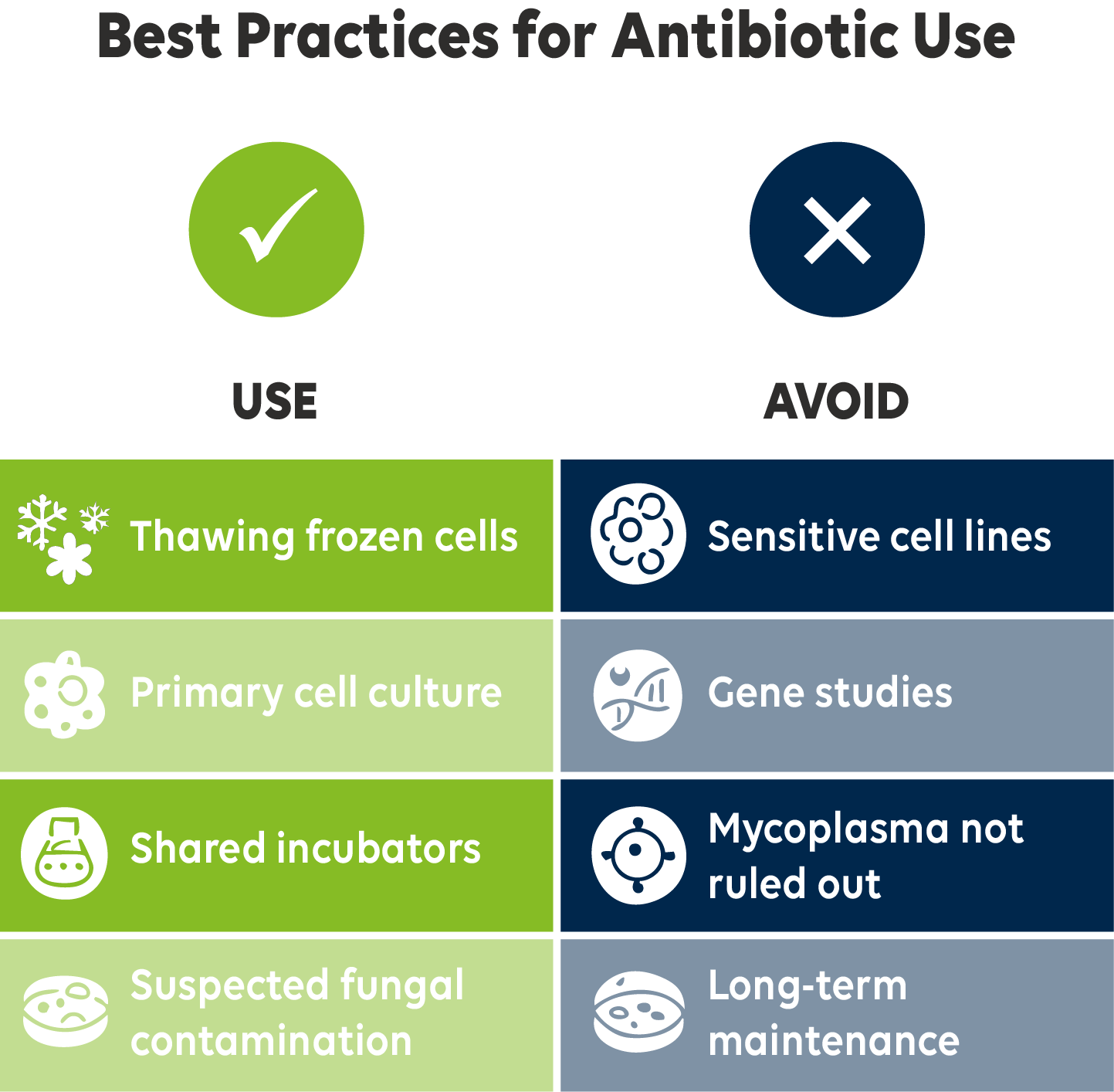
| Scenario | Recommended Approach | Why |
|---|---|---|
| Thawing frozen cells | Use antibiotics | Cells are vulnerable during initial recovery. |
| Primary cell culture (early passages) | Use antibiotics | Reduces the risk of early loss due to contamination. |
| Shared incubators or crowded lab settings | Use antibiotics (short term) | Increased potential for contamination. |
| Suspected fungal or mixed contamination | Use antibiotic-antimycotic combo | Broad coverage, but suitable only for short-term use. |
|
|
||
| Sensitive cell types (e.g., stem cells) | Avoid antibiotics | More susceptible to cytotoxic and off-target effects. |
| Gene expression, epigenetic, or phenotype studies | Avoid antibiotics | These compounds can alter cellular behavior and skew results. |
| Mycoplasma not ruled out | Avoid antibiotics | May suppress symptoms — use targeted detection and treatment instead. |
| Long-term maintenance of clean cultures | Avoid antibiotics | Can mask aseptic issues and promote resistance over time. |
In antibiotic-free cultures, contamination reveals itself quickly — and that’s a good thing. You need to know when something’s wrong.
For a broader overview of contamination risks beyond antibiotics, see our guide on 5 Common Sources of Cell Culture Contamination and How to Prevent Them.
Related Products
- Penicillin/Streptomycin (100x) – Broad-spectrum base antibiotic for Gram-positive/negative coverage.
- Antibiotic/Antimycotic Solution (100x) – Combines Pen-Strep with Amphotericin B for fungal and bacterial protection.
- MycoXpert, Mycoplasma Removal Reagent (50x) – Targeted reagent to eliminate mycoplasma contamination in culture.
Conclusion
Antibiotics are not inherently bad and harmful — but relying on them by default creates more problems than it solves. In the long run, strong technique and regular contamination monitoring do more to protect your cultures than any antimicrobial blend.
Use antibiotics with intent, not out of habit — and you'll preserve both your cell lines and the reliability of your data.
Ready to rethink your antibiotic strategy?
Download our lab-ready PDF with decision tables, checklists, and working concentrations — perfect for your next experiment or SOP revision.
Download the PDF GuideFrequently Asked Questions (FAQs)
What is the difference between Pen-Strep and Gentamicin?


Pen-Strep acts synergistically against both Gram-positive and Gram-negative bacteria. Gentamicin has broader coverage, especially for Gram-negative strains, but it’s more cytotoxic — particularly in sensitive cell types.
Can I use antibiotics to eliminate mycoplasma?


Not reliably with standard antibiotics. Mycoplasma lacks a cell wall, making it inherently resistant to most conventional agents. However, dedicated elimination reagents — often based on targeted antibiotic formulations — can be effective when combined with proper detection and follow-up testing. PCR screening remains essential for confirming success.
Learn more about Mycoplasma contamination in our Knowledge Center.
Is antibiotic-antimycotic solution 100× the same everywhere?


Most follow a similar formula (Pen-Strep-Amphotericin), but concentrations and stabilizers vary by supplier. Always check the specifications.
Should I stop using antibiotics entirely?


Not necessarily. Many labs reduce routine use but keep antibiotics for high-risk steps like thawing or early passages. The key is to use them intentionally — not by default — and always alongside proper aseptic technique.
Are there antibiotics that don’t affect gene expression?


Almost all antibiotics can interfere with cellular pathways. If gene expression is critical, it’s best to skip antibiotics entirely and monitor your cultures closely.
Can I reduce antibiotic concentrations to lower cytotoxicity?


While it’s technically possible to reduce concentrations, this should only be done temporarily and with extreme caution. Sub-inhibitory doses may promote resistance and are not recommended for routine use.
What’s the best way to check for contamination without antibiotics?


To detect hidden contamination, inspect cells under the microscope, run PCR-based mycoplasma tests, or use DAPI staining — and consider culturing without antibiotics for 1–2 passages to uncover suppressed infections.
Learn more about Mycoplasma testing in our Knowledge Center.
Related Articles
Mycoplasma Contamination: Everything you should know
Learn how Mycoplasma silently alters cell culture data, how to detect it early, and how to eliminate it effectively with MycoXpert.
Learn how Mycoplasma silently alters cell culture data, how to detect it early, and how to eliminate it effectively with MycoXpert.
Cell Culture Contamination: 5 Common Sources and How to Prevent Them
Identify and prevent the five most frequent threats to your cell culture—bacteria, fungi, mycoplasma, viruses, and cross-contamination—with clear visuals and lab-ready tips.
Identify and prevent the five most frequent threats to your cell culture—bacteria, fungi, mycoplasma, viruses, and cross-contamination—with clear visuals and lab-ready tips.