
Cytogenetic Media & Reagents
for Pre- and Post-Natal Diagnostics
Cytogenetics is essentially the study of chromosomes, particularly during mitosis/meiosis. It investigates how genetics play a crucial role in cellular processes and therefore in the development and further progression of diseases.
Consequently, cytogenetic media and reagents are of utmost importance for diagnostics to maintain an excellent patient care. Depending on the issue to be investigated, cytogenetic diagnostics can be further divided into pre-natal and post-natal diagnostics.
PRE-NATAL
Pre-natal diagnostics focus on the analysis of, e.g., amniotic fluid (amniocentesis) or chorionic villi cells.
POST-NATAL
Post-natal diagnostics focus on the analysis of, e.g., peripheral blood lymphocytes or bone marrow cells.
CYTOGENETIC REAGENTS
Reagents are needed for the analysis of cells, e.g. to arrest cells in metaphase during mitosis or to stimulate mitosis.
Quick Links
What is Cytogenetics?
Traditionally, the term "cytogenetics" was merely used to describe the investigation of chromosomal abnormalities and how they are linked to a certain disease. For example, in the 1960s, scientists discovered the chromosomal defect causing the Down syndrome. Since then, numerous diseases have been linked to chromosomal abnormalities.
Nowadays, the depth of this field could be further expanded by the term "molecular cytogenetics", which includes – besides the classic karyotyping method – various applications of molecular biology, such as fluorescence in situ hybridization (FISH) or comparative genomic hybridization (CGH).
These methods offer more accurate means of discovering regions on the chromosome that can be linked to a certain medical condition. Furthermore, it has become an essential means for cancer diagnostics and to determine the way a patient may respond to a specific therapeutic.
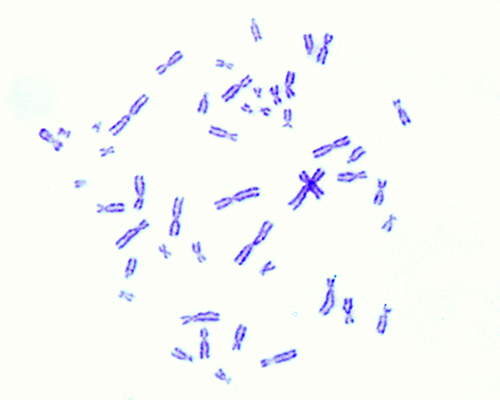
Karyotyping and Chromosomal Anomalies
The process of pairing and ordering all the chromosomes of an organism is referred to as karyotyping, the final image – that reveals potential aberrations – is referred to as a karyogram. The observations to be made are ample and include:
- size and count of the chromosomes
- position of the centromeres
- distribution of heterochromatic regions
- other structural abnormalities
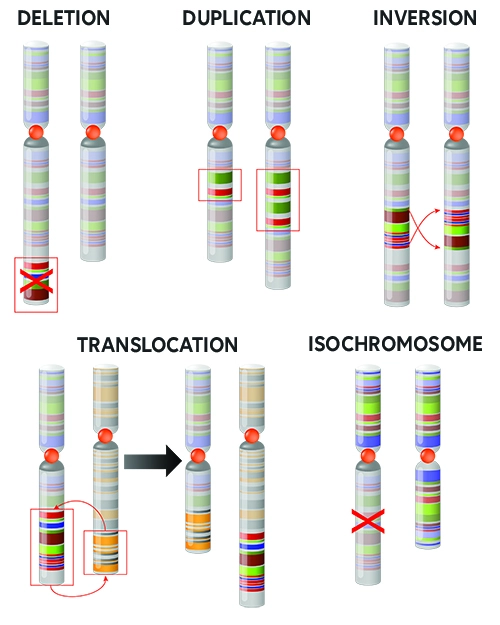
Some examples of structural abnormalities can be seen in the picture on the right, such as deletion, duplication, or inversion.
Most of these occurrences are derived directly from the egg or sperm cell and therefore can be found in the entire organism. In some cases, the abnormality takes place later in development and is therefore only found in a specific cell lineage of the body.
Molecular Cytogenetics
Whereas in early approaches of cytogenetics, scientists merely had staining and microscoping techniques at hand, the more recent term molecular cytogenetics includes numerous molecular biology applications, such as:
- Fluorescence in situ hybridization (FISH)
- Spectral Karyotyping (SKY)
- Multicolor FISH (M-FISH)
- Comparative Genomic Hybridization (CGH)
Generally speaking, these techniques enable the detection of specific nucleic acid sequences in morphologically preserved chromosomes. This way, even chromosomal abnormalities that involve small segments of DNA, can be revealed.
Cytogenetic diagnostics can be divided into two major sectors: pre-natal and post-natal. Whereas pre-natal diagnostics – as the name already suggest – focus on chromosomal abnormalities to be observed before birth, post-natal diagnostics find their application at any age after birth.
Pre-natal cytogenetics
Pre-natal diagnostics are often applied when certain pregnancy risk factors are present, such as a family history of genetic disorders, pregnancy during later years (over 35), high blood pressure, diabetes, asthma, etc. The aim is to identify chromosomal abnormalities in the early stages of fetal development, in order to evaluate the possibility of pregnancy termination.
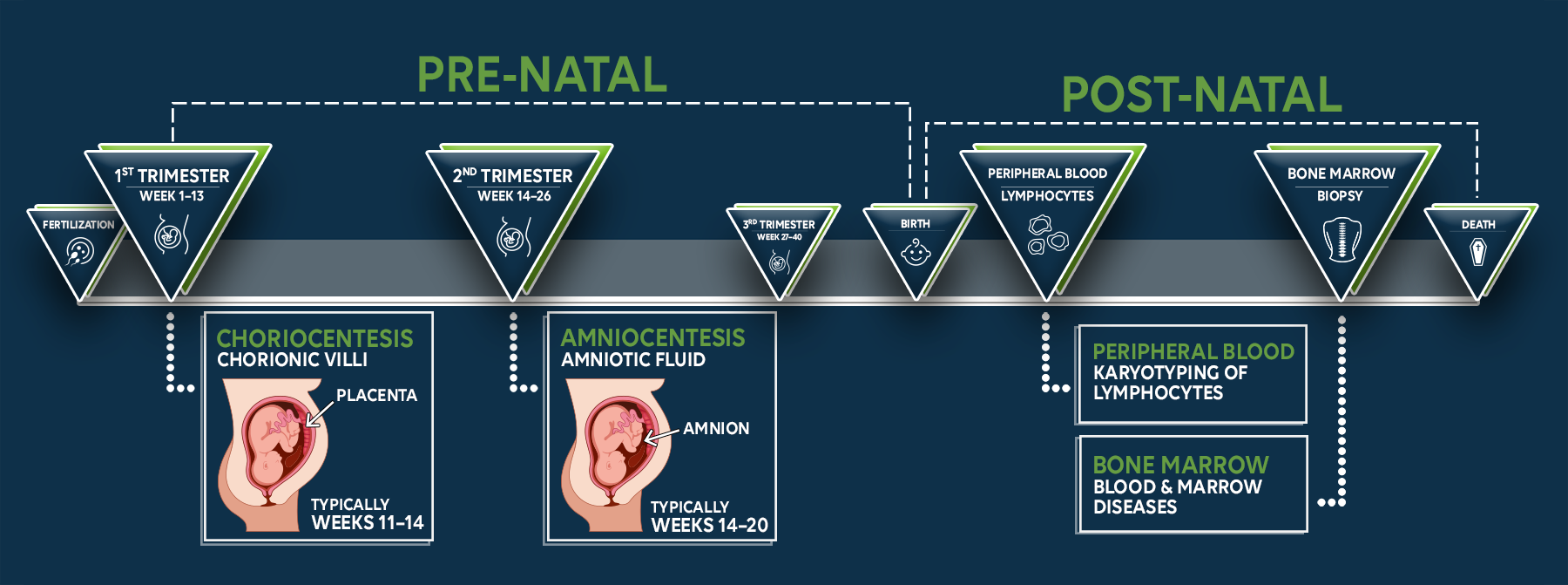
Post-natal cytogenetics
Post-natal cytogenetic diagnostics include numerous methods, yet the main players are the analysis of hematopoietic cells (blood-forming cells) and peripheral blood lymphocytes.
Hematopoietic cells are obtained by bone marrow aspiration, which is a very common procedure in hematology. These cells can develop into any type of blood cell, and the analysis aims to give healthcare providers additional insights of how to treat a certain condition, such as leukemia. For example, a disrupted gene resulting in an altered cellular function is often the cause of tumorigenesis. The further investigation of this gene and how the alteration takes place can now lead to the discovery of potential treatment opportunities.
The analysis of blood lymphocytes is also an important tool to gain more information on chromosomal abnormalities. Apart from offering data from a different angle in the blood cell lineage, it can show how genetic disorders are spread among a population and how they are linked to a certain phenotype.
Simply put, a blood sample is more easily obtained than a bone marrow aspirate, offering a time-saving and undemanding approach. Nevertheless, lymphocytes sourced from bone marrow remain an important player in cytogenetics, as blood lymphocytes are already differentiated.
Regardless of the diagnostic method, the process usually involves the steps shown below. In the end, a photographic image of the arranged chromosomes – a karyogram – is obtained.
First of all, a sample is taken from the patient, e.g., via choriocentesis, amniocentesis, blood sample, or bone marrow aspiration.

Next, the primary sample is grown in a cell culture until a sufficient amount of cells are present – depending on the application.
The cultured cells are arrested with help of a mitostatic agent, such as colcemid or colchicin.
The mitostatic agent prevents spindle formation during mitosis, causing the cells to arrest in metaphase.
The cells are harvested, treated with a hypotonic solution (e.g., potassium chloride), and stained.
Cell staining can be done by numerous means, e.g., Giesma dye.

After staining, the metaphase chromosomes are now cleary visible under the microscope.
A photographic image is taken and rearranged to identify and pair the chromosomes.

The final image is referred to as a karyogram.
After analysis, the healthcare provider can now evaluate chromosomal abnormalities and recommend what the best course of action is.
Cytogenetic Media
Pre-natal diagnostics
Amniotic Fluid and Chorionic Villi
When it comes to pre-natal diagnostics, regardless of whether you are testing the amniotic fluid or chorionic villi, the time it takes until a result is obtained is of vital importance. Not only are the parents often anxiously waiting on the results – depending on the outcome, termination of pregnancy may be an option that needs to be considered.
For analysis, a significant amount of metaphase chromosomes is necessary. Therefore, the cells obtained by sampling must first be cultured – as quickly as possible. The medium that you choose should be optimized for the tissue type and support rapid cell growth.
Post-natal diagnostics

Peripheral Blood Lymphocytes
In routine testing of peripheral blood lymphocytes, you'll want to go for an optimized, time-saving growth medium that stimulates lymphocyte growth.
Hence, the addition of Phytohemagglutinin-M (PHA-M) is essential for the short-term cultivation of lymphocytes. This is because lymphocytes do not normally undergo subsequent cell divisions – a mitogen, such as PHA-M, is required to induce mitosis.
Bone Marrow and Leukemic Blood Cells
Even though you could analyze bone marrow samples directly for chromosomal abnormalities, it is necessary to first grow them in cell culture. This is because for some bone marrow samples, the disorder will only show after subsequent cell divisions in culture.
When culturing bone marrow or leukemic blood samples, different hematopoietic cells (myeloid and lymphoid lineages) are present. Therefore, depending on the cell type you want to promote, different growth stimulants are required (please see "Instructions for Use" for more information). Regardless, general growth-promoting factors for bone marrow and leukemic blood samples should be present in your medium.
Cytogenetic Reagents

Phytohemagglutinin M (PHA-M)
Phytohemagglutinin (PHA) is a mitogen that is used for the stimulation of cell proliferation in lymphocytes and monocytes, and the agglutination of erythrocytes. It is a lectin found in legumes and most commonly extracted from the red kidney bean (Phaseolus vulgaris).
PHA actually consists of two closely related molecules: leucoagglutinin (PHA-L) and erythroagglutinin (PHA-E), and each of these consist again of five isolectines (tetrameres) that are held together by non-covalent forces. PHA-M is the mucoprotein form and is a crude extract – it contains all the solutes extracted from the plant material.
Colcemid
Colcemid, also known as Demecolcine, is closely related to the natural alkaloid colchicine, but it is less toxic. It is commonly used in cell culture to arrest cells in metaphase for karyotyping, particularly for lymphocyte karyotyping
Colcemid acts by depolymerizing microtubules and inhibiting spindle fiber formation. In medical applications, it is also used to improve cancer therapies by synchronizing tumor cells in metaphase, where the cells are most sensitive to radiotherapy.
Potassium Chloride (KCl)
Potassium Chloride (KCl) is commonly used for various cytogenetic applications, including karyotyping, fluorescence in situ hybridization (FISH), and chromosome analysis.
Due to the lower osmotic pressure of KCl in comparison to cell plasma, this solution can create an osmotic pressure difference that makes it suitable as a lysing reagent. In this process, it causes the cell’s membranes to swell and rupture, exposing the chromosomes for subsequent analysis.
In general, treating cells with KCl facilitates their expansion for optimal spreading of metaphase chromosomes during karyotyping procedures. After treatment, chromosomes can be fixed and stained using various dyes to visualize the banding patterns, allowing cytogeneticists to analyze their structure and identify any abnormalities under the microscope.
Frequentlsy Asked Questions (FAQ)
Can I still use my products if they arrive in a thawed state?


Yes! We recommend a controlled thawing process at 4 °C in the refrigerator. Afterward, you need to homogenize the solution by gently swirling the bottle. You can now aliquot the medium and refreeze it.
Important: Only apply the controlled thawing process if you can ensure that your product is at least 50 % frozen (visible volume) – otherwise we advise you to discard the product as the performance can no longer be guaranteed.
How should the media be stored after use?


Once opened, the products can be stored at +2 °C to +8 °C and should be used within the specified period:
- AmnioPrime: 2 weeks
- MarrowPrime: 2 weeks
- LymphoPrime and LymphoPrime 2: 10 days
- PHA-M: 4 weeks
- Colcemid: 4 – 6 weeks
If unopened, you can store the media at -15 °C until the expiration date.
Do I need to add further supplements to the media?


No, our media are delivered ready to use and do not require any further supplementation with additives.
On what components is your formulation based?


AmnioPrime and MarrowPrime:
The formulation is based on a MEM-Alpha basal medium with the addition of gentamicin, L-glutamine, serum, hormones, and growth factors.
LymphoPrime and LymphoPrime 2:
The formulation is based on a basal medium with addition of gentamicin, L-glutamine, serum, antibiotics, and PHA-M.
Do the media contain animal substances?


Yes, we add pre-tested fetal bovine serum (FBS) to our media.
Which medium is best suited for chromosomal analyses?


MarrowPrime:
Supports cell attachment and growth of bone marrow and leukemic blood cells.
LymphoPrime and LymphoPrime 2:
Both LymphoPrime media are best suited for short-term cultivation of lymphocytes from peripheral blood.
AmnioPrime:
AmnioPrime is specifically designed for the cultivation of human primary amniotic and chorionic villus cells intended for karyograms, fluorescence in situ hybridization (FISH), and other cytogenetic methods. It provides optimal growth conditions for amniocytes (amniotic fluid cells) and chorionic villus cells (placenta).
My PHA-M is appearing cloudy. Can I continue to use the product?


PHA-M may appear turbid at +2 °C to +8 °C. The turbidity does not affect the activity of PHA-M.
What is the difference between colchicine and Colcemid?


N-deacetyl-N-methycolchicine (Colcemid) and colchicine are related alkaloids. Among other uses, they are used for mitosis inhibition, suppressing the formation of spindle fibers by binding to free receptors. The main difference between the two products is that Colcemid has fewer toxic properties compared to colchicine.
My Colcemid exhibits precipitate formation. Can this affect my analyses?


As soon as precipitate formation is visible, we recommend that you to stop using the product.
How much PHA-M do I need to add to get a concentration of 10 µg/ml?


After thawing, each milliliter contains 5 to 10 mg of protein. We recommend using 2 to 4 ml of PHA-M per 100 ml of your karyotyping medium.
My media is showing a color change, can I continue to use the product?


Often a color change of the media is associated with a pH shift. During production, the media are adjusted to their specific pH values. Transport on dry ice or storage for a longer period of time may cause a deviation from the original pH value. In this case, we recommend incubating the affected bottles with the screw cap slightly open. To do this, turn the cap slightly (approx. ¼ turn) and incubate the bottle for approx. 1 hour at 37 °C and 5% CO2 in a cell culture incubator. This allows the pH value to readjust.
My media have a differing color gradient in the frozen state. Can I continue to use the media?


A final statement about the quality of the media can only be made in the thawed state. We recommend that you thaw the media using a controlled thawing process (4 °C in the refrigerator) and then gently swirl the media. In many cases, the apparent color difference disappears, and the product shows its usual color.
Are your products also suitable for therapeutic use?


No, our products are only suitable for use in research and for in vitro diagnostics. Our products are not approved for therapeutic procedures on humans and animals.
What quantities of material are required for testing?


Amniotic fluid cells after amniocentesis: 10 – 15 ml amniotic fluid.
Chorionic villus cells after chorionic villus sampling (CSV): 15 – 20 mg chorionic villus tissue
Postnatal chromosome analysis on lymphocytes: 2 – 10 ml whole blood
Postnatal chromosome analysis on tissue samples: 1 cm² skin biopsy, umbilical cord
Instructions for Use
In situ culture of amniotic fluid cells


- Concentrate the cells by centrifugation of the amniotic fluid: Centrifuge 20 ml of amniotic fluid at 750 rpm for 10 minutes.
- Carefully decant the amniotic fluid from the cell pellet into a sterile test tube.
- Resuspend the cell pellet with 2 ml of amniotic fluid. Add 2 ml of AmnioPrime medium and swirl gently.
- Culture 0.5 ml of the cell suspension on each coverslip in a tissue culture dish.
- Incubate cultures at +37 °C in a 5 % CO2 atmosphere.
- Add 2 ml of AmnioPrime medium to each culture on day 2.
- Check cultures for growth after 4 to 5 days. Feed cultures once growth has been observed. To feed cultures, carefully aspirate all the exhausted culture medium and replace it with 2 ml of fresh AmnioPrime medium. Recommendation: feed cultures every 2 days.
- When the cultures have colonies of sufficient size, proceed with harvesting. For best results, feed cultures with AmnioPrime medium the day before the harvest.
Flask method culture of amniotic fluid cells


Use the same procedure as for the in situ culture, with the following adaptations:
- Resuspend the cell pellet with 4 ml of amniotic fluid. Add 16 ml of AmnioPrime medium and swirl gently.
- Culture 5 ml per T25 flask. Incubate undisturbed at +37 °C in a 5 % CO2 atmosphere.
- Check all flasks for growth after 5 days. For best results, feed cultures with AmnioPrime medium the day before the harvest.
Recommendations for closed systems:
Method 1: Supplement AmnioPrime medium with 2 % (v/v) sterile 1 M HEPES solution. The HEPES solution must be set to pH 7.0 at +20 °C. HEPES-supplemented medium can subsequently be used on cells in closed culturing flasks.
Method 2: Pre-equilibrate the flask containing AmnioPrime medium and cells at +37 °C in a 5 % CO2 atmosphere for 1 hour prior to closing the flask.
Method 3: Flush each culture flask containing AmnioPrime medium and cells with 5 % CO2 – 95 % air through 0.2-µm sterile filter for 20 seconds. Tighten the caps and incubate the flasks at +37 °C.
Harvesting protocol for bone marrow cells


- Tubes are centrifuged for 5 minutes at 1500 rpm.
- Remove supernatant.
- Resuspend pellet in 6 ml of pre-warmed potassium chloride solution (KCl, 0.075 M) and incubate tubes at +37 °C in a water bath for 20 minutes.
- Centrifuge tubes at 1500 rpm for 5 minutes.
- Remove supernatant.
- Add 5 ml of fixative (3 methanol: 1 acetic acid) to the tube. Slowly add a few drops of fixative, mixing gently. Continue adding fixative in this way until all cell clumps have disintegrated, and the cell suspension is as homogeneous as possible.
- Centrifuge at 1500 rpm for 5 minutes.
- Repeat steps 6 – 7 twice.
- After the last washing step, carefully remove supernatant without affecting the pellet. Resuspend pellet in appropriate volume of fixative for slide preparation.
Protocol for setting up and culturing bone marrow cells


- If a bone marrow sample is received in transport medium, centrifuge at 150 to 170 × g for 10 minutes. For bone marrow sample received in heparin, go directly to step 3.
- Carefully remove the supernatant, including any fat and debris floating on the surface, and discard. Do not affect the pellet.
- Place 5 ml of MarrowPrime medium into each tube.
- Seed with the appropriate amount of bone marrow cells using sterile Pasteur pipettes. The final concentration of cells should be 106 cells/ml per culture.
- Set up cultures according to provisional diagnosis:
Direct culture: Add 100 µl of Colcemid Solution (10 µg/ml) for 1 to 2 hours. Short term culture: Incubate overnight. The following morning, add 100 µl of Colcemid Solution (10 µg/ml) for 1 to 2 hours. Overnight exposure to Colcemid: Add 50 µl (10 µg/ml) of Colcemid Solution as late in the day as possible. Incubate overnight at +37 °C. Short term culture + overnight exposure to Colcemid: Incubate at +37 °C for 24, 48 or 72 h. Then add 50 µl (10 µg/ml) of Colcemid solution as late in the day as possible. Incubate overnight at +37 °C B-cell stimulated cultures: Add 100 µl PMA (4-phorbol 12-myristate 13-acetate) and/or PWM (pokeweed mitogen) and incubate for 2 to 4 days at +37 °C. Add 100 µl of Colcemid solution (10 µg/ml) and incubate overnight at +37 °C. T-cell stimulated cultures: Add 100 µl PHA (phytohaemagglutinin) and incubate 72 hours at +37 °C. Add 100 µl of Colcemid solution (10 µg/ml) for 1 to 2 hours.
How to prepare a hypotonic solution (0.075 M KCl) for karyotyping


This protocol shows you how to make 1 liter of 0.075 M KCl solution.
- Weigh in 5.6 g KCl
- Fill up to 1000 ml with distilled water
Please note: the hypotonic solution should be pre-warmed to 37 °C before use.
LymphoPrime & LymphoPrime 2: Culture of peripheral blood lymphocytes for chromosome analysis


- Thaw LymphoPrime medium and make aliquots of 10 ml (sterile tubes).
- Thaw the precalculated amount of LymphoPrime medium (in tubes) until room temperature is reached.
- Transfer 0.5 ml of heparinized whole blood into a tube containing 10 ml LymphoPrime medium.
- Incubate the culture at +37 °C, 5 % CO2 in an incubator for 72 h.
- Add 0.1 – 0.2 ml of Colcemid Solution (Cat. No. COL-H) to each culture tube (at a final concentration of 0.1 µg/ml). Incubate the culture for an additional 15 – 30 min.
- Transfer the culture to a centrifuge tube and spin at 500 × g for 5 mi.
- Remove the supernatant and resuspend the cells in 5 – 10 ml of hypotonic 0.075 M KCl, pre-warmed to +37 °C. Incubate at +37 °C for 10 – 12 min.
- Spin at 500 × g for 5 min.
- Remove the supernatant, agitate the cellular sediment and add 5 – 10 ml of fresh, ice-cold fixative drop by drop, made up of 1 part acetic acid to 3 parts methanol. Leave at + 4 °C for 10 min.
- Repeat steps 8 and 9.
- Spin at 500 × g for 5 min.
- Resuspend the cell pellet in a small volume (0.5 – 1 ml) of fresh fixative, drop onto a clean slide and allow to air dry.
- At this stage, the preparation can be stained with Giemsa. For Giemsa staining, the most widely used method, you can use one of the common staining protocols or the protocol established in your laboratory.
Using PHA-M without AmnioPrime: Culture of peripheral blood lymphocytes for chromosome analysis


- Add 2 – 4 ml of PHA-M per 100 ml of karyotyping medium.
- Transfer 0.5 ml of heparinized whole blood into a tube containing 10 ml karyotyping medium supplemented with PHA-M (or 106 viable cells per ml).
- Incubate the culture at +37 °C, 5 % CO2 in an incubator for 72 hours.
- Add 0.1 – 0.2 ml of Colcemid Solution (Cat. No. COL-H) to each culture tube (at a final concentration of 0.1 µg/ml). Incubate the culture for an additional 15 – 30 min.
- Transfer the culture to a centrifuge tube and spin at 500 × g for 5 min.
- Remove the supernatant and resuspend the cells in 5 – 10 ml of hypotonic 0.075 M KCl, pre-warmed to +37 °C. Incubate at +37 °C for 10 – 12 min.
- Spin at 500 × g for 5 minutes.
- 8. Remove the supernatant, agitate the cellular sediment and add 5 – 10 ml of fresh, ice-cold fixative drop by drop, made up of 1 part acetic acid to 3 parts methanol. Leave at +4 °C for 10 min.
- 9. Repeat steps 7 and 8.
- 10. Spin at 500 × g for 5 min.